Menaquinol binding site (HQNO as ligand)
HQNO is used as the analogue substrate, which has an amide oxide instead of a hydroxide group. It is also missing a methyl group. Such analogue is used to obtain crystallography data of qNOR conformation upon electron donor ligand binding, as it allows stable binding required for a formation of uniform lattice structure.Below is the picture of menaquinol binding site
Residues involved:
Asp746 à
As carboxylate, it forms H-bond with hydroxyl group of HQNO
His328 à
Side chain forms H-bond with amine oxide of HQNO and another H-bond with Glu332
that connects to the extracellular side.
Phe336 and Phe337 à
Hydrophobic interaction with polycarbon tail
As menaquinol is oxidised to form menaquinone, its two protons are released into the extracellular side, probably through His328, Glu332 H-bond network, whereas its electrons are donated to Fe core in heme b. Distance between Fe at heme b to bound HQNO is 10Å.
As menaquinol is oxidised to form menaquinone, its two protons are released into the extracellular side, probably through His328, Glu332 H-bond network, whereas its electrons are donated to Fe core in heme b. Distance between Fe at heme b to bound HQNO is 10Å.
Calcium ion binding
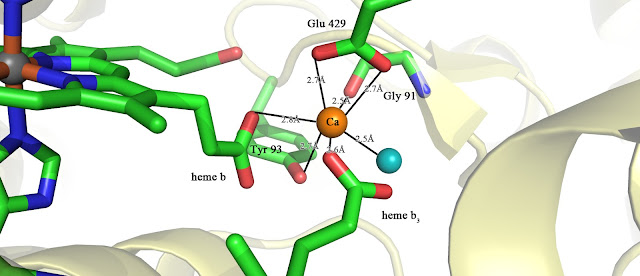
The two electrons are transferred to the second heme b3 that forms the binuclear centre through the bridging calcium ion. The picture is presented below
The calcium is ligated by Tyr93, Glu429, Carbonyl oxygen of Gly91, Water, and
propionate groups (C2H5COO-) of the heme b and heme b3.
Function of calcium ion is presumably vital due to decrease in enzyme activity upon side-chain substitution of Tyr93 or Glu429. Apart from bridging the electron transfer, its possible functions are:
Function of calcium ion is presumably vital due to decrease in enzyme activity upon side-chain substitution of Tyr93 or Glu429. Apart from bridging the electron transfer, its possible functions are:
- May maintain protein conformation as it is located between transmembrane and hydrophilic domain
- Regulate redox potential of heme irons
HTML Comment Box is loading comments...